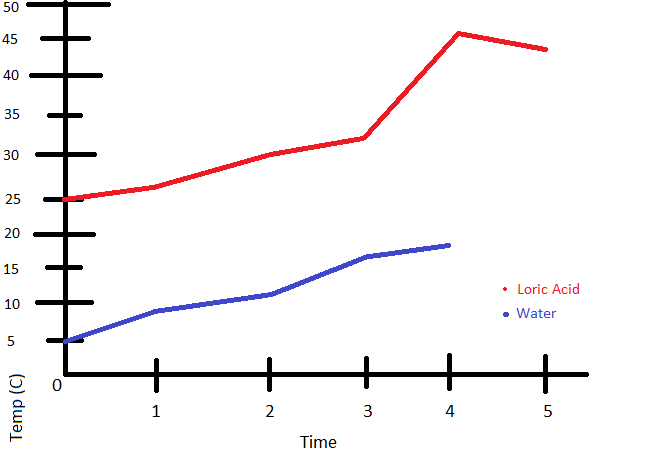
The initial temperature of an empty pop can is 20 degrees celcius. The pop can is heated and is then places in a cold environement. This causes the temperature of the gas inside the pop can to change to 12 degrees celcius.
What is the final volume of the air inside the pop can? (v of 12oz can=355mL (v=355mL))
The same pop can in the previous example starts under normal atmospheric pressure. This is 100.325 kilopascals.
What is the final pressure inside the pop can?
P1*V1=P2*V2
P2=P1*V1/V2
=(100325)(355)/
What are 6 common phase changes?
Melting, freezing, vaporization, condensation, sublimation, and deposition are six common phase changes
Which are exothermic and which are endothermic?
Endothermic: Melting, vaporization, sublimation
Exothermic: Freezing, condensation, deposition
What is the heat of fusion?
The energy needed to melt
What is the heat of vaporization
The energy to turn liquid into a gas
I'm gona get at least a B because I didnt study as much as I normally do. However, I have this down mostly and I should be able to get a B
Hands-on activities help me learn better than taking notes, but notes also help me learn
1.) C
2.) A
3.) B
4.) A
5.) A
6.) A
7.) C
8.) C
9.) B
10.) A
11.) They are too small to actually see
13.) It.. um... idk ._.
15.) ... no
17.) how the actual heck would i know :v
19.) .-.
21.) -_-
23.) It moves around a lot? Idk
He discovered it by putting magnets by crokes when experimenting with them
The 3 subatomic particles are electrons, neutrons, and protons.
Electrons are negatively charged (-), neutrons are neutrally charged (0), and protons are positivly charged (+)
Their mass - electrons are much smaller than protons and neutrons.
| Element | # of P+ | # of E- | # of N0 |
|---|---|---|---|
| Nickel | 28 | 28 | 31 |
| Arsenic | 33 | 33 | 42 |
| Sulfur | 16 | 16 | 16 |
| Oxygen | 8 | 8 | 8 |
| Element | # of P+ | # of N0 |
|---|---|---|
| Rb-88 | 37 | 51 |
| N-18 | 7 | 11 |
| Cl-40 | 17 | 23 |
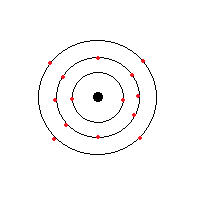
Look back in your book or your notes at Dalton's atomic theory and think about what we have learned sice that time. Use what you know to write your own "modern atomic theory"
An atom has a nucleus contains heavy protons and neutrons that is in the middle. Around it is a "cloud" of electrons that spin around it. It cannot be known at any time in particular where an electron specifically is. Electrons are negatively charged, protons are positively charged, and neutrons have no charge.